In this article we will be reviewing studies that investigate the impact of optimal testosterone on metabolic markers such as fasting glucose, fasting insulin, HbA1c, and triglycerides (explaining the importance of these markers as we go). We will look at a meta-analysis, one study from this meta-analysis and then a large study on using testosterone for the treatment and prevention of type 2 diabetes. As we dissect this literature we will also look at optimal dosing regimens and why some of the studies do this sub optimally.
Physiology and Importance of Testosterone Replacement Therapy (TRT) on Metabolic Parameters: Insights from a Meta-Analysis
Study Overview- 2014 meta-analysis
A 2014 meta-analysis (Cai et al.) examined the impact of testosterone replacement therapy (TRT) on several metabolic parameters: fasting glucose, fasting insulin, HbA1c, and triglycerides in diabetic men. The studies involved various forms of testosterone administration—gel, oral, and intramuscular injections—with a mean follow-up of approximately seven months. I’ve synthesized the results and put them in to context with a some explanation on the markers.
I’ve picked out the Heufelder et al (2009) study (a study from the meta-analysis) as it was one of the larger studies, that was conducted over a longer period (52 weeks). I like this study as the participants were given a daily dose of testosterone (gel), which is consistent and therefore biologically more similar to how testosterone is released in the body. Further to this all participants were placed on a nutrition and exercise programme (which should be the case). In this study, thirty-two hypogonadal men (testosterone <12.0 nmol/L) with newly diagnosed type 2 diabetes and metabolic syndrome were enrolled. Sixteen received daily testosterone gel (50 mg) in combination with supervised diet and exercise, while the other 16 received diet and exercise alone. At the end of the 12 months, average testosterone levels went up from 10.4 nmol/L to 11.2 nmol/L (in the non testosterone group) and from 10.5 to 15.4 nmol/L (in the testosterone group) bioavailable testosterone increased from 4.3 nmol/L to 5.5 nmol/L(in the non testosterone group) and from 4.5 nmol/L to 8.1 nmol/L in the testosterone group.
Fasting Glucose
Physiological Background: Fasting glucose reflects the body’s baseline blood sugar levels, primarily maintained by the balance between hepatic glucose production and tissue glucose uptake, mediated by insulin and glucagon. Abnormal fasting glucose levels are indicators of insulin resistance, diabetes, or metabolic dysfunction.
Findings from Meta-Analysis
- Across all forms of testosterone, the aggregate analysis demonstrated a statistically significant mean reduction in fasting glucose by 1.10 mmol/L (approximately 20 mg/dL).
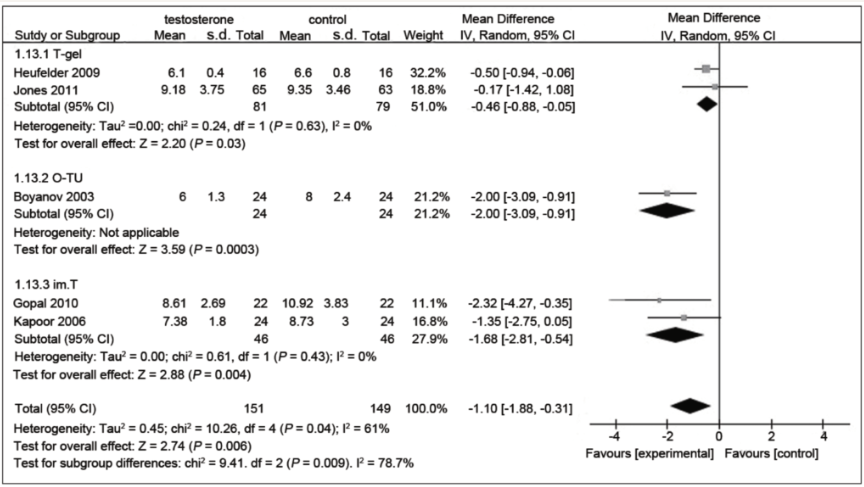
Figure 1. Focussing in on the right hand side of this chart (commonly used in meta-analysis), we can see at the bottom, the bottom diamond is left of the line, showing a statistically significant (evidenced based) drop in fasting glucose. (Cai et al. 2014)
Fasting Insulin
Physiological Background: Fasting insulin reflects pancreatic beta-cell function and tissue insulin sensitivity. Elevated fasting insulin indicates insulin resistance and compensatory hyperinsulinemia, precursors to type 2 diabetes.
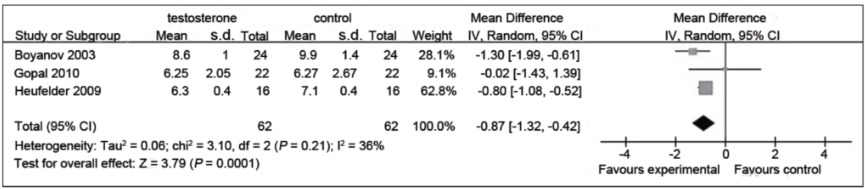
Figure 2: A favourable impact on fasting insulin levels that was statistically significant. (Cai et al. 2014)
Findings from Meta-Analysis
- Most studies showed a trend toward reduced fasting insulin levels with TRT.
HbA1c (%)
Physiological Background
HbA1c reflects average blood glucose levels over the previous two to three months. It is a cornerstone marker for monitoring diabetes and assessing long-term glycemic control.
Findings from Meta-Analysis
- Two of the three studies included in the analysis showed a reduction in HbA1c.
- The overall change was nearly 1% in HbA1c reduction.
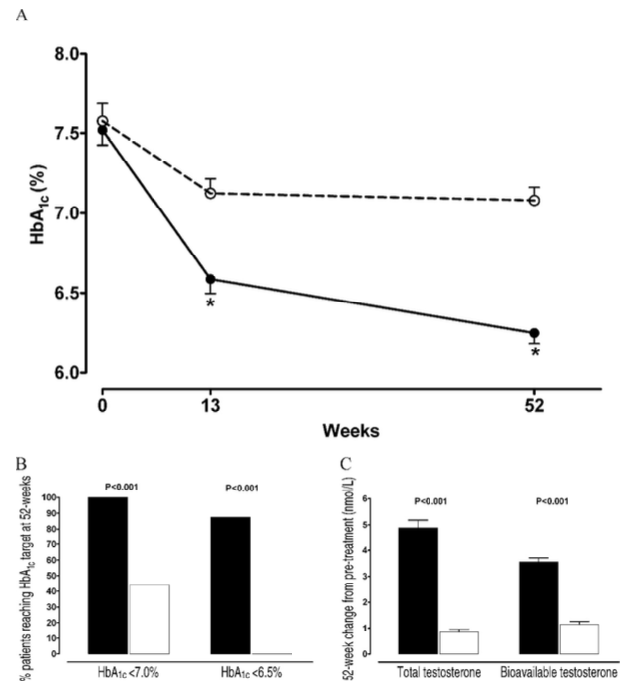
Figure 3: Glycemic Control and Testosterone Profiles (A) HbA1c levels throughout the study. White circles: supervised diet and exercise only; black circles: diet, exercise, and testosterone gel.
(B) Percentage of patients achieving HbA1c <7.0% (left) and <6.5% (right). White boxes: diet and exercise alone; black boxes: diet, exercise, and testosterone gel. (C) Changes in total and bioavailable testosterone after 52 weeks. White boxes: diet and exercise only; black boxes: diet, exercise, and testosterone gel. Data are shown as mean ± SE. * P < .001. Heufelder et al (2009)
Clinical Implication
A nearly 1% reduction in HbA1c can significantly lower the risk of diabetes complications. TRT appears to benefit glycemic control in men with low testosterone levels and coexisting diabetes or prediabetes.
Triglycerides
Physiological Background
Triglycerides are a form of fat stored for energy. Elevated levels are a marker of metabolic syndrome and a risk factor for cardiovascular disease.
Findings from Meta-Analysis
- A modest reduction in triglyceride levels was observed with TRT, although the magnitude of change varied across individual studies.
- The improvements were not as pronounced as those seen for fasting glucose or HbA1c.
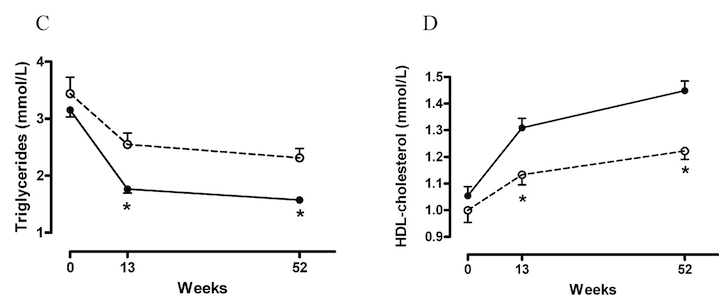
Figure 4: A more robust drop in triglycerides from the Heufelder et al (2009) study (down almost a third)
Clinical Implication
While TRT’s effect on triglycerides is less robust, the modest improvement may still contribute to better cardiovascular risk profiles, particularly in individuals with hypertriglyceridemia.
Summary of Meta-Analysis Results
The 2014 meta-analysis demonstrates that testosterone replacement therapy offers measurable metabolic benefits, particularly in reducing fasting glucose and HbA1c levels. While the impact on fasting insulin and triglycerides is less pronounced, the observed trends suggest potential improvements in insulin sensitivity and lipid profiles. These findings reinforce the value of TRT as a therapeutic option for men with low testosterone and metabolic disturbances, though individual results may vary based on the method of administration and baseline metabolic status.
In addition the Heufelder et al (2009) study showed improvements in waist circumference, adiponectin (a vital hormone released by fat) and hsCRP (key serum markers of insulin sensitivity) and hepatic steatosis (fat around the liver). I’ve written before about research that indicates fat around the organs (pancreas and liver) being a main driver in diabetes. Removing this fat has been proven to reverse diabetes— in previous studies this has been done by very low calorie diets. However testosterone offers perhaps a more promising strategy to remove this fat, alongside calorie restriction, by maintaining muscle mass and reducing fat mass.
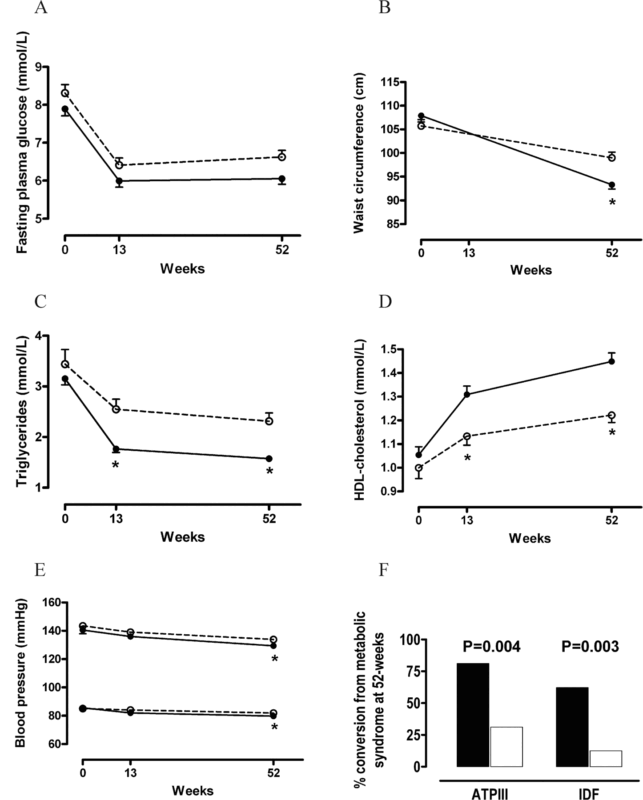
Figure 5: Components of the metabolic syndrome and metabolic syndrome conversion rate during the course of the study. (A) Fasting plasma glucose, (B) waist circumference, (C) triglycerides, (D) high-density lipoprotein (HDL) cholesterol, (E) systolic and diastolic blood pressure, and (F) metabolic syndrome conversion rate after 52-week treatment with supervised diet and exercise alone (white circles and white boxes) or in combination with transdermal testosterone administration (black circles and black boxes) Heufelder et al (2009)
2021 Study: Testosterone Treatment to Prevent or Reverse Type 2 Diabetes in Men Enrolled in a Lifestyle Program (T4DM)
Study Design:
This was a large 2021 study that investigated the efficacy and safety of testosterone treatment in men with impaired glucose tolerance or newly diagnosed type 2 diabetes. The men also had a serum testosterone concentration of 14·0 nmol/L or lower but without pathological hypogonadism.
The aim was to explore whether testosterone can prevent the progression of impaired glucose tolerance to type 2 diabetes and potentially reverse newly diagnosed type 2 diabetes, in addition to the effects of a lifestyle intervention. In this case, the lifestyle intervention used was Weight Watchers.
This was a randomized, double-blind, placebo-controlled trial, conducted over two years. Men aged 50 to 74 were included in the study and received intramuscular testosterone replacement therapy (TRT).
Concerns with the dosing regimen used in the Study:
Participants received 1000 mg of intramuscular testosterone at baseline, again six weeks later, and then every three months for the next two years. It can be argued that a more optimal dose would be 100 mg weekly, noting that the high doses used in the study could result in “super-physiologic” levels of testosterone, which would likely diminish in effectiveness by the end of the dosing cycle due to the testosterone’s half-life (The half-life of testosterone cypionate when injected intramuscularly is approximately eight days)
Primary Outcomes:
The primary outcomes of the study included two-hour glucose levels following an oral glucose tolerance test (OGTT). Researchers focused on participants whose two-hour glucose levels were at or above 11.1 mmol/L (200 mg/dL), a level historically used to diagnose type 2 diabetes.
At the two-year mark, 21% of participants in the placebo group reached this threshold, compared to 12% in the testosterone group, reflecting a 40% reduction, which was statistically significant. The main takeaway from this study is the significant reduction in the number of participants who triggered a diabetes diagnosis.
Results at Two Years:
- Placebo group: 21% reached the threshold for diabetes.
- Testosterone group: 12% reached the threshold for diabetes.
- This 40% reduction in diagnosis rates was statistically significant.
Secondary Outcomes:
In terms of secondary outcomes, the study showed:
- A decrease in waist circumference by nearly one inch.
- An increase in total muscle mass by 1.7 kg (about 4 lbs).
- A reduction in fat mass by approximately 6 lbs.
- A 2.5% reduction in abdominal fat percentage.
- A small increase in arm muscle mass.
- A 5 lb improvement in muscle strength in the non-dominant hand.
Conclusions and closing thoughts
All the studies here, and others looked at, show a clearly favourable impact on metabolic health parameters from optimising testosterone. Considering the tidal wave of diabetes and the mortality risk associated with diabetes, such as cardiovascular disease, the clinical implications of optimising testosterone are huge. This is even more understated in aging men, as maintaining muscle mass is vital. Infact maintaining muscle mass seems to be one of the mechanisms by which testosterone impacts insulin sensitivity. The elegance of testosterone signalling is that it maintains muscle mass by removing fat from around the organs, which is vital for paracrine glucose homeostasis.

