Want to catch up with the other articles in this series?
Part 1: What is the impact of endurance training on human lifespan?
Part 1.1: What is VO2max?
Part 2: What are training zones?
Part 2.1: How do muscle fiber types correspond to training zones?
Part 2.2: Training zones 1, 2 and 3
Part 2.3: Training zones 3, 4, 5 and 6
Part 2.4: Moderate intensity vs. high intensity exercise
Part 3: By which mechanisms does exercise delay aging and prevent chronic disease?
Part 3.1: What are the cardiovascular adaptations to endurance training?
Part 3.2: What are the metabolic adaptations to endurance training?
Part 3.3: Can exercise prevent or even treat cancer?
Part 3.4: How does exercise impact the immune system?
Part 3.5: Exercise as a therapy for neurodegeneration and conclusions
What are the metabolic adaptations to endurance training?
Mitochondria are the metabolic powerhouses of the cell that produce energy in the form of ATP. Challenging the mitochondria through endurance exercise leads to longer term benefits such as mitochondrial biogenesis and increased mitochondrial density. We can think of this as analogous to hypertrophy of type IIx muscle from resistance training. However, when we exercise in zone 2 and above, we are improving the quality and capacity of type I and II fibers.
By training regularly, the body becomes not only a more adept fat burning machine but less susceptible to aging. This is partially explained by the presence of mitochondrial dysfunction in the pathogenesis of chronic diseases with significant mortality rates.
The total mass of our mitochondria can be considered the human engine. The bigger and more efficient this engine is at burning fat the fitter and healthier we are as humans.
Mitochondrial homeostasis
Mitochondria in parallel with muscle mass, adapt to the demands placed on them i.e. they have extraordinary plasticity. They can either grow and improve if we demand it or they shrink and become faulty with inactivity. The choice is ours.
Exercise puts stress on the cellular environment which leads to biological signals instructing our cells to increase the capacity of the body to produce energy through our mitochondria. The generation of new mitochondria, otherwise known as biogenesis, is an adaptation leading to increased exercise performance or V̇O2 max. It facilitates increases in aerobic capacity and ability to perform in aerobic conditions through generating more ATP.
Similarly, the process of joining mitochondria to make them bigger is known as fusion, resulting in a bigger aerobic capacity.
The aging mitochondria
As humans age, there is a decline in the function of mitochondria. This decline in mitochondrial function may contribute to the development of certain age-related diseases. The hallmarks of aging mitochondria include:
- a decrease in activity of mitochondrial enzymes
- a decrease in respiratory capacity per mitochondria
- an increase in the production of reactive oxygen species
One of the most significant age-related changes is the progressive loss of muscle mass, known as sarcopenia.
This muscle loss is caused by structural and molecular changes within the muscle tissue, and it has significant implications for mobility, the risk of falls, and the onset of type 2 diabetes and obesity.
Mitochondria play an important role in the progression of sarcopenia. They are important regulators of a variety of factors that contribute to the pathogenesis of sarcopenia, such as ATP provision, oxidative stress, proteostasis, apoptosis, inflammation, and calcium handling.
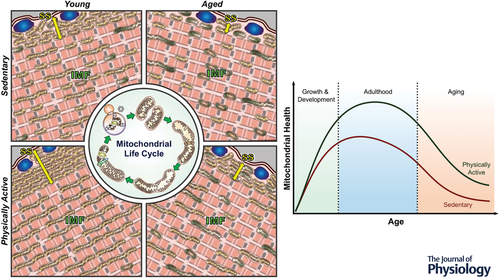
The natural process of aging along with chronic inactivity leads to an increase in impaired or damaged mitochondria, further contributing to the progressive nature of sarcopenia. Many studies have shown that the number of mitochondria decreases with age. The expression of PGC-1α, a protein that is considered the master regulator of mitochondrial protein expression, also decreases with age and inactivity. This probably contributes to the reductions in mitochondrial volume. PGC-1α levels are reduced in aging muscle as a consequence of decreased transcription of the PGC-1α gene.
A central issue regarding mitochondrial health in aging muscle is whether the apparent decline in function and content is a result of increasing levels of physical inactivity, or a consequence of the aging process. Regular exercise has been shown to delay this decline in function and induce mitochondrial adaptations that match a younger phenotype (version).
This indicates that regular exercise can help to preserve mitochondrial function and mitigate the effects of aging on muscle mass and function.
Exercise induces mitochondrial remodelling and delays aging
Regular exercise training can lead to a number of adaptations in our muscles and mitochondria that can improve their response to nutrients and increase their capacity for energy production. The type of exercise and intensity can influence the specific adaptations that occur. For example, moderate-intensity exercise in a carbohydrate-restricted state can promote the use of fat as a fuel source and lead to increases in the enzymes responsible for fat metabolism.
Exercise has been shown to lead to an increase in mitochondrial content, as well as an increase in enzymes involved in oxidative phosphorylation and the respiratory capacity per mitochondrion. Additionally, training can reduce the production of reactive oxygen species (ROS), which are associated with cellular damage and aging. Endurance exercise training can increase total mitochondrial proteins, including those involved in beta-oxidation, the tricarboxylic acid cycle (TCA cycle), and the electron transport chain— improving the capacity for energy provision to the exercising muscle
Exercise can also reduce the accumulation of mitochondrial ROS and reduce apoptotic cell death in muscle. It can also increase the expression levels of many of the proteins that are involved in energy metabolism of aging muscle, restoring the ATP synthesis capability of the mitochondria.
Prolonged endurance training can increase the mitochondrial volume by 40-50%. It has been observed that people who have maintained high physical activity throughout their life have preserved mitochondrial health, and those who take up targeted training at any age, including middle to late years, are capable of restoring mitochondrial health to more closely resemble that of their younger selves.
Exercise is therefore considered as the most powerful (non-pharmacological) strategy for the maintenance of metabolic health into the late stages of life.
Cellular mechanisms of exercise induced mitochondrial adaptations
Exercise induces important, tangible changes in mitochondrial content and quality. These changes are beneficial for metabolic health and reduce aging of the mitochondria.
These adaptations are favourable for improved muscle health. Scientists have strived to determine the precise signalling pathways mediating mitochondrial quality control, which includes the result of biogenesis and fusion, as opposed to fission and mitophagy.
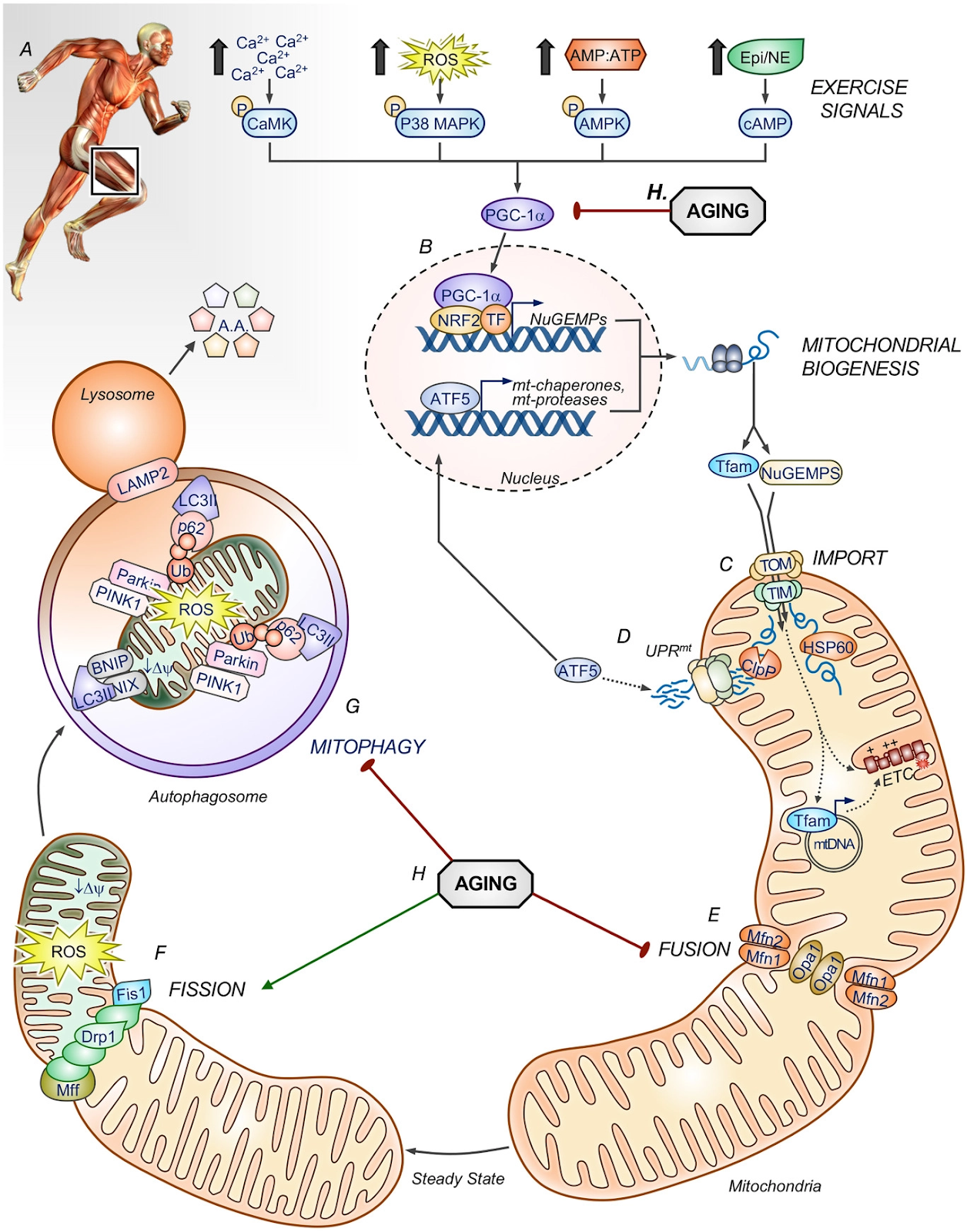
The mechanisms by which this happens is summarised below and corresponds to letters A – H as shown in the figure.
A: Exercise signals activate muscle fibres, this leads to:
- Muscle contraction: myosin-actin interactions, crossbridge cycling, and the hydrolysis of ATP.
- Activation of AMPK: Increased hydrolysis of ATP produces AMP activating an important metabolic regulator AMPK. This protein is responsible for sensing the energy status of the cellular environment and initiating corresponding metabolic pathways.
- Increased reactive oxygen species during exercise
- Increased hormonal signalling (adrenaline, cortisol) activating cAMP.
B: Activation of PGC-1α: these cellular processes activate the signalling kinases that converge on PGC-1α, allowing it to travel to the nucleus. In the nucleus of the cell thai protein expresses and produces proteins that will be involved in mitochondrial fusion and fission — promoting their repair and recycling.
C: Mitochondrial biogenesis: The process of creating new mitochondria.
D: Upregulation of proteins to help with metabolic processes.
E: Increased mitochondrial fusion: the physical merging of the outer and then the inner mitochondrial membranes of two originally distinct mitochondria producing a bigger more powerful organelle.
F: Decreased mitochondrial fission: dividing mitochondria are associated with cellular degradation.
G: Increased mitophogy: the process of clearing and recycling damaged mitochondria.
H: The Impact of ageing on the above processes. Disruption of PGC-1α signalling and mitochondrial biogenesis (red line), as well as an increased fission to fusion ratio (green line). This creates a fragmented network and disrupts mitophagy.
Mitophagy and autophagy with exercise
Endurance exercise both optimises important metabolic processes and piles on mitochondrial mass.
It’s just as important for the body to recycle and repair damaged mitochondria. This process is known as mitophagy. This is similar to ‘autophagy‘ which can be literally translated as ‘self eating’. These processes are linked to a variety of longevity benefits.
They occur when a cellular recycling ‘bin’ or ‘truck’ known as an autophagosome engulfs damaged or old structures, breaks them down and removes them from the cell.
Mitophagy is accelerated by cellular stress, such as a lack of ATP brought about by exercise.
During exercise, the AMP/ATP ratio increases, activating AMPK and its downstream target Unc-51 like autophagy activating kinase 1 (ULK1). At the same time mTORC1, a known suppressor of the process, is inhibited (shown in figure 3.4)
Research has documented increases in the apparatus of mitophagy as an adaptation in response to regular exercise.
Lactate clearance and mitochondrial health
Lactate is present in the blood at all times and mitochondria are responsible for lactate clearance. Type I muscle fibres (slow twitch fibres) have large amounts of mitochondria to perform this task. Exercise remodels these mitochondria, improving their ability to clear lactate. Blood lactate level is not only a marker of mitochondrial function but overall metabolic health.
Poor mitochondrial function results in an inability to clear lactate. The mitochondria are damaged and lack the apparatus to clear lactate. High resting blood levels are associated with a variety of diseases including diabetes and metabolic syndrome— diabetes, high blood pressure and cardiovascular disease.
Lactate is an omnipresent product of metabolism. Traditionally we consider lactate a bad thing. However emerging research is identifying lactate as fuel rather than a toxin. It is the blood acidity or H+ that is correlated with rises in lactate that leads to symptoms of extreme fatigue.
At increasing levels of exercise intensity, a specific transporter called MCT-4, exports lactate from type II fibers. Type I muscle fibers play a key role in the clearance of lactate . Type I muscle fibres contain a transporter called MCT-1 which takes up lactate and transports it to the mitochondria where it is reused as energy.
Targeted exercise increases MCT-1 transporters— high performing individuals can produce high levels of output with low levels of blood lactate (under 2 mmol/l of lactate levels) in response to exercise. The capacity to clear lactate is associated with better metabolic health and increased exercise capacity.