In this article we will be covering:
- Mental health in men and it’s significant impact on mortality
- Theories of depression and limitations of traditional models
- Testosterone and mood regulation: the biological connection
- A review of a recent meta-analysis investigating the relationship between testosterone and depression
Intro
Depressive disorders, including major depressive disorder (MDD) and dysthymic disorder, are significant mental health challenges, characterized by persistent low mood, negative thoughts, and chronic fatigue. These disorders have profound personal and societal consequences, with the World Health Organization (WHO) recognizing depression as the leading cause of disability worldwide. Despite the availability of various pharmacological and psychotherapeutic treatments, around 30% of individuals with depression do not achieve sustained remission, even after multiple interventions. This treatment gap has spurred interest in exploring alternative therapies, including hormonal approaches like testosterone therapy, particularly for men.
Here we will investigate the biological mechanisms of depression, and how testosterone fits into those mechanisms. This helps to gain a better understanding of the biochemistry and endocrinology of testosterone and its effects on the brain and body. We will then go on the look at testosterone in clinical practice for men in the context of depression.
Mental Health in Men and All Cause Mortality
Suicide is a leading cause of death in men. Research shows that mental illness is the most important risk factor for suicide. More than 90% of people who die by suicide struggle with their mental health and/or addiction. Researchers estimate that up to 60% of people who die by suicide have major depression, this is a huge problem.
- Canada: Suicide is the 2nd leading cause of death for men under the age of 50
- UK: Suicide is the single largest cause of death for men under the age of 50
- USA: Suicide is the 2nd most common cause of death for men under the age of 45
Mechanisms of Depression
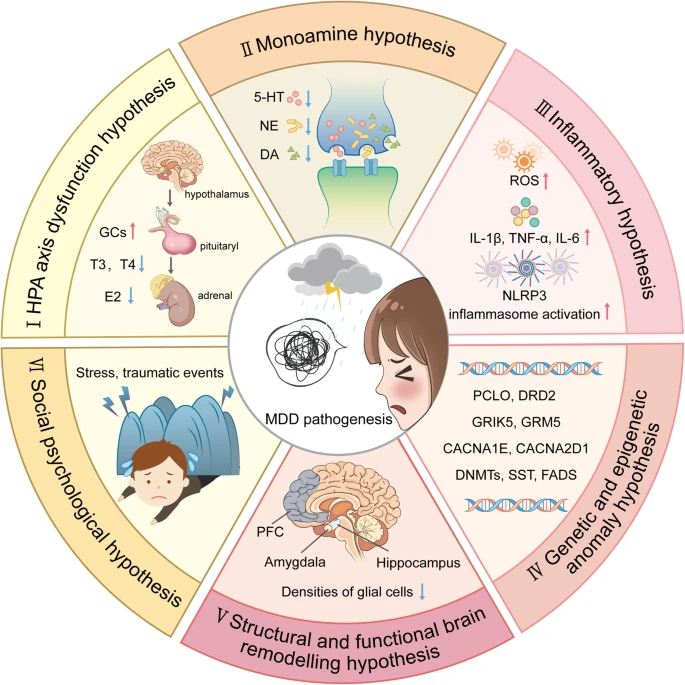
Figure 1: An Outline Map of Hypotheses Explaining MDD Pathogenesis (Wang, S. et al. 2024)
- (I) HPA Axis Dysfunction Hypothesis: Elevated glucocorticoids (GCs) are central to MDD’s development. Both thyroid hormone (TH) and estrogen influence HPA axis functionality. Chronic stress leads to dysregulation of the Hypothalamic-Pituitary-Adrenal (HPA) axis and glucocorticoid resistance in immune cells, resulting in an overactive immune response. This response includes the release of Damage-Associated Molecular Patterns (DAMPs) and subsequent signaling through Pattern Recognition Receptors (PRRs), creating a feedback loop that escalates both peripheral and central inflammation.
- (II) Monoamine Hypothesis: MDD primarily results from deficiencies in serotonin (5-HT), dopamine (DA), and norepinephrine (NE). However a recent meta analysis showed serotonin research provides no consistent evidence of there being an association between serotonin and depression, and no support for the hypothesis that depression is caused by lowered serotonin activity or concentrations. (Moncrieff, J. et al 2023)
- (III) Inflammatory Hypothesis: Neuro-inflammation, driven by reactive oxygen species (ROS), inflammatory cytokines, and inflammasome activation, is implicated in MDD’s onset. Peripheral inflammation contributes to central inflammation by compromising the blood-brain barrier, facilitating immune cell migration into the brain, and activating glial cells. Activated glial cells release substances that disrupt neurotransmitter balance, neural plasticity, and circuit function, with microglial activation being particularly significant.
- (IV) Genetic and Epigenetic Anomaly Hypothesis: Genetic predispositions in MDD patients include genes like PCLO (presynaptic vesicle trafficking), DRD2 (dopamine receptor D2), GRIK5 and GRM5 (glutamate receptors), CACNA1E and CACNA2D1 (calcium channel subunits), DNMTs (DNA methyltransferases), SST (somatostatin transcription), and FADS (fatty acid desaturase).
- (V) Structural and Functional Brain Remodeling Hypothesis: Postmortem studies in MDD patients reveal decreased glial cell density in key areas like the prefrontal cortex (PFC), hippocampus, and amygdala. MRI studies often reveal reduced hippocampal volumes in these patients. There’s also evidence of neural circuitry dysfunction, notably hypoactivation between the ventral striatum and ventromedial prefrontal cortex, which is linked to melancholic depression.
- (VI) Social Psychological Hypothesis: Traumatic or highly stressful life events significantly increase the risk of developing MDD.
Testosterone and Mood Regulation: The Biological Connection
Testosterone, is a neuroactive steroid hormone and exerts a significant influence on mood regulation and neural function. A growing body of research has investigated the potential of testosterone therapy to alleviate depressive symptoms, offering promising but nuanced findings.
Testosterone’s impact on mood is rooted in its influence on brain chemistry.
- One of its key effects is the enhancement of serotonin release, particularly in the dorsal raphe nuclei, a critical brain region for mood stabilization. Serotonin, a neurotransmitter commonly targeted by antidepressant medications, plays a vital role in emotional regulation and overall well-being.
- Additionally, testosterone promotes neuroplasticity, the brain’s ability to form new neural connections. This adaptability is essential for individuals to overcome entrenched patterns of negative thinking and emotional distress, a core challenge in depressive disorders.
Research using animal models has demonstrated that testosterone administration can enhance serotonin activity and facilitate antidepressant-induced neuroplasticity, providing a biological foundation for its mood-enhancing effects. These findings align closely with the mechanisms of action observed in traditional antidepressants, suggesting that testosterone may serve as a complementary or alternative treatment approach for certain individuals.
In a review on molecular actions of sex hormones in the brain and their potential treatment use in anxiety disorders the discussion highlighted the complex and context-dependent role of steroid sex hormones (SSHs), particularly focusing on their effects on anxiety-like behavior in males:
- Rodent studies reveal that testosterone (T) and its metabolites, such as dihydrotestosterone (DHT) and estradiol (E2), play key roles in modulating anxiety. These effects vary by brain region, with significant anxiolytic-like actions observed in the hippocampus (HIP). T’s influence on anxiety is mediated primarily through androgen receptor (AR) signaling, with additional contributions from the serotonergic system, GABA A receptor pathways, and BDNF/TrkB signaling.
In male rodents, T-derived metabolites such as DHT and E2 demonstrate notable anxiolytic effects, suggesting that the conversion of T to active metabolites may be critical for its impact on behavior. The hippocampus, a region heavily implicated in mood regulation, appears to be a central site for these effects. Prenatal exposure to high levels of T also shows sex-specific outcomes, with male offspring exhibiting a reduced risk of anxiety-like behaviors compared to females, indicating a protective effect in males.
Clinical studies have started exploring the therapeutic potential of steroid sex hormones in male populations. The administration of T, alone or in combination with traditional antidepressants like imipramine, has shown promising effects on reducing anxiety symptoms. These findings align with preclinical research and suggest that T supplementation might be a viable treatment option for anxiety disorders in men, particularly those with low baseline T levels.
Additionally, the combination of T with other pharmacological agents holds potential. For instance, pairing T with existing antidepressants may enhance treatment outcomes by leveraging T’s neuromodulatory properties. Such strategies could offer a more targeted approach, addressing specific pathways implicated in male anxiety, such as AR signaling and serotonergic system modulation.
Despite these advances, the discussion underscores the importance of personalized approaches to steroid sex hormones-based therapies. Factors such as age, health status, hormone concentration, and individual differences in genetic predisposition can significantly influence treatment efficacy. For males, these considerations are especially relevant given the intricate interplay between T levels, mood regulation, and anxiety.
Evidence from Clinical Studies: Insights from Meta-Analysis
Meta-analysis link: Walther A, Breidenstein J, Miller R (2018)
The relationship between testosterone therapy and depression has been examined in numerous randomized controlled trials (RCTs) and meta-analyses, shedding light on its potential benefits. A comprehensive meta-analysis of 27 RCTs, encompassing nearly 1,890 participants, provides some of the most compelling evidence to date. This analysis revealed that testosterone treatment was associated with a modest but statistically significant reduction in depressive symptoms compared to placebo, with a standardized effect size (Hedges’ g) of 0.21.
In practical terms, this effect translates to an approximate 2.2-point reduction in the Beck Depression Inventory-II (BDI-II) score, a widely used measure of depressive symptoms. This improvement is particularly meaningful when considering that the National Institute for Health and Care Excellence (NICE) guidelines suggest a reduction of 2 to 3 points as clinically significant for depression treatments. Notably, testosterone therapy’s efficacy in reducing depressive symptoms is comparable to many established antidepressant medications, especially when used as an adjunct treatment.
The meta-analysis also identified several subgroups of men who appeared to benefit most from testosterone therapy. These included middle-aged men, men with low baseline testosterone levels (hypogonadal), and those with mild depressive symptoms. In addition, men with coexisting conditions such as HIV or treatment-resistant depression showed promising improvements. Importantly, the analysis demonstrated that testosterone’s beneficial effects were not confined to hypogonadal men; even eugonadal men (with normal testosterone levels) experienced reductions in depressive symptoms.
Clinical Implications for Men’s Mental Health
The findings from clinical research and meta-analyses highlight testosterone therapy’s potential as a valuable tool in managing depressive symptoms in men. Its dual action in—modulating brain chemistry and promoting neural adaptability—makes it uniquely suited to address the biological and psychological dimensions of depression. Testosterone therapy’s demonstrated efficacy in alleviating emotional distress, increasing energy, and improving overall quality of life further underscores its potential.
For men who have not fully responded to conventional antidepressant therapies, testosterone therapy may offer a complementary approach. By targeting underlying hormonal imbalances or enhancing mood-regulating pathways, it provides a more holistic treatment strategy. Additionally, its potential to improve associated symptoms such as low libido, fatigue, and diminished motivation offers broader benefits for overall well-being.
Expanding Understanding: Implications for Research
While testosterone therapy has shown promise in addressing depressive symptoms, further research is needed to fully understand its role and refine treatment protocols. The meta-analysis highlighted key areas for exploration, such as identifying optimal dosages and durations for therapy. The analysis also revealed that higher doses of testosterone (>500 mg/week) were associated with more robust improvements, suggesting the need for dose-response studies to establish precise guidelines.
The timing of therapeutic effects also warrants investigation. Studies have reported varying timelines for the onset of benefits, ranging from six weeks to several months. Clarifying these time frames could help clinicians better monitor progress and adjust treatment plans accordingly. Additionally, future research should examine the combined effects of testosterone therapy with psychological or behavioral interventions, potentially creating synergistic benefits for patients.
Bridging Gaps in Men’s Mental Health
Testosterone therapy’s potential to address depressive symptoms represents an important step forward in tackling the mental health challenges faced by men. Depression often manifests differently in men, with symptoms such as irritability, anger, and physical complaints sometimes overshadowing emotional distress. As a result, men are less likely to seek help or receive accurate diagnoses, leading to untreated or poorly managed conditions.
By integrating testosterone therapy into the broader spectrum of mental health care, clinicians can provide a more tailored approach to men’s needs. This strategy not only addresses hormonal imbalances but also helps combat the stigma surrounding mental health treatment by framing it within the context of overall physical and emotional well-being.
Conclusion
Depressive disorders, notably Major Depressive Disorder (MDD) and dysthymic disorder, pose significant challenges to mental health, particularly in men where suicide remains a leading cause of death. The traditional models for understanding depression, like the monoamine hypothesis, have been questioned by recent research, including a meta-analysis which found no consistent evidence linking serotonin levels directly to depression. This has led to an exploration of alternative mechanisms and treatments, including the role of testosterone.
Testosterone, beyond its well-known roles in physical health, has a profound impact on mood regulation through its influence on brain chemistry, enhancing serotonin release and promoting neuroplasticity. A comprehensive meta-analysis involving 27 randomized controlled trials has shown that testosterone therapy can significantly alleviate depressive symptoms, with effects comparable to some established antidepressants. This is particularly beneficial for middle-aged men, those with low baseline testosterone, and individuals with treatment-resistant depression or coexisting conditions.
The biological connection between testosterone and mood regulation involves complex pathways, including the modulation of anxiety-like behavior through androgen receptor signaling, serotonergic systems, and neurotrophic factors like BDNF. Clinical studies have indicated that testosterone supplementation can be an effective treatment for men with anxiety disorders, especially when combined with traditional antidepressants.
However, the application of testosterone therapy for mental health is not without considerations. Dosage, duration, and individual patient factors such as age, baseline hormone levels, and genetic predispositions must be carefully evaluated to optimize treatment outcomes. This personalized approach could significantly bridge the gap in managing depression and associated symptoms like low libido and fatigue, which are often underdiagnosed or poorly addressed in men.
In conclusion, while testosterone therapy holds great promise for improving mental health outcomes in men, particularly by addressing the biological underpinnings of depression, it also calls for further research to refine treatment protocols, understand long-term effects, and integrate this therapy with psychological interventions. By doing so, we can enhance our approach to men’s mental health, offering more tailored and effective solutions to combat one of the leading causes of disability worldwide.

